|
|
 |
|
|  |
|
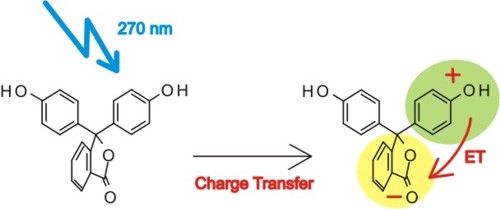
Ultrafast photoinduced intramolecular charge transfer (CT) in triphenylmethane lactones
is investigated for the first time by femtosecond pump-probe spectroscopy.
The studied class of molecules, lactonic forms of triarylmethane dyes
(triphenylmethane lactones) are relatively simple molecular systems with
donor (D) and acceptor (A) units built around a tetrahedral carbon atom.
As demonstrated by spectroscopic experiments in the group of Dr. Jerzy Karpiuk such an
arrangement of D and A results in a weak electronic coupling in the ground state
and the absorption transitions are localized on their structural subunits. In the
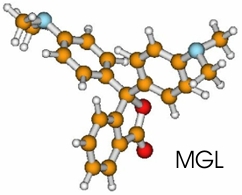
case of malachite green lactone (MGL) the donor moiety is one of the two dimethylaniline
(DMA) groups while in the case of phenolphthalein (PP) it is one of the two phenol
rings. The chromophore which accepts the electron is the phthalide moiety (Pd) in both studied
lactones.
The molecules are investigated in different aprotic solvents
such as acetonitrile, ethyl acetate and n-hexane. Thus the influence of the solvent
polarity as well as of solvation processes is determined.
|
|
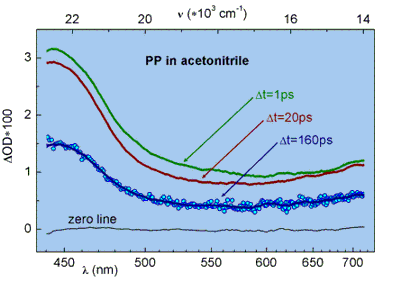
Transient absorption spectra of the molecules in different solvents are measured with a white light continuum at several delays
after optical excitation at 270 nm. The transient spectra of PP and MGL consist mainly of a broad absorption band with a maximum at 440 nm
and 465 nm, respectively. The band decays exponentially on a ns-time scale and can be described to the absorption band of the phenol
radical cation in PP and to the DMA radical cation in MGL. The appearance of the radical cation absorption band is a direct evidence for the
ultrafast photoinduced charge transfer process in both studied lactones.
|
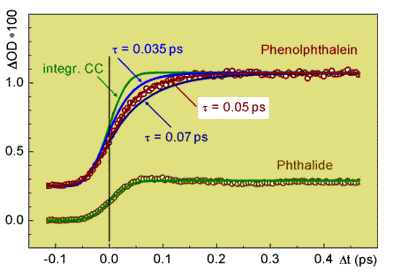
The build-up of the cation radical absorption band describes the kinetics of the charge transfer in MGL and PP.
After optical excitation of the lactones at 270 nm it is directly monitored at a probe wavelength of 475 nm with a time resolution
of 60 fs. In PP in acetonitrile the phenol radical cation appears with a rise time of 50 fs. This is the fastest electron transfer
directly observed so far for organic intramolecular D-A systems.
|
|
|  |
"The key role of solvation dynamics in intramolecular electron transfer: time resolved photophysics of crystal violet lactone"
U. Schmidhammer, U. Megerle, S. Lochbrunner, E. Riedle, J. Karpiuk
J. Phys. Chem. A112, 8487 - 8496 (2008)
Details
"50-fs Photoinduced Intramolecular Charge Separation in Triphenylmethane Lactones"
T. Bizjak, J. Karpiuk, S. Lochbrunner, and E. Riedle
J. Phys. Chem. A 108, 10763 - 10769 (2004)
Details
| | |

